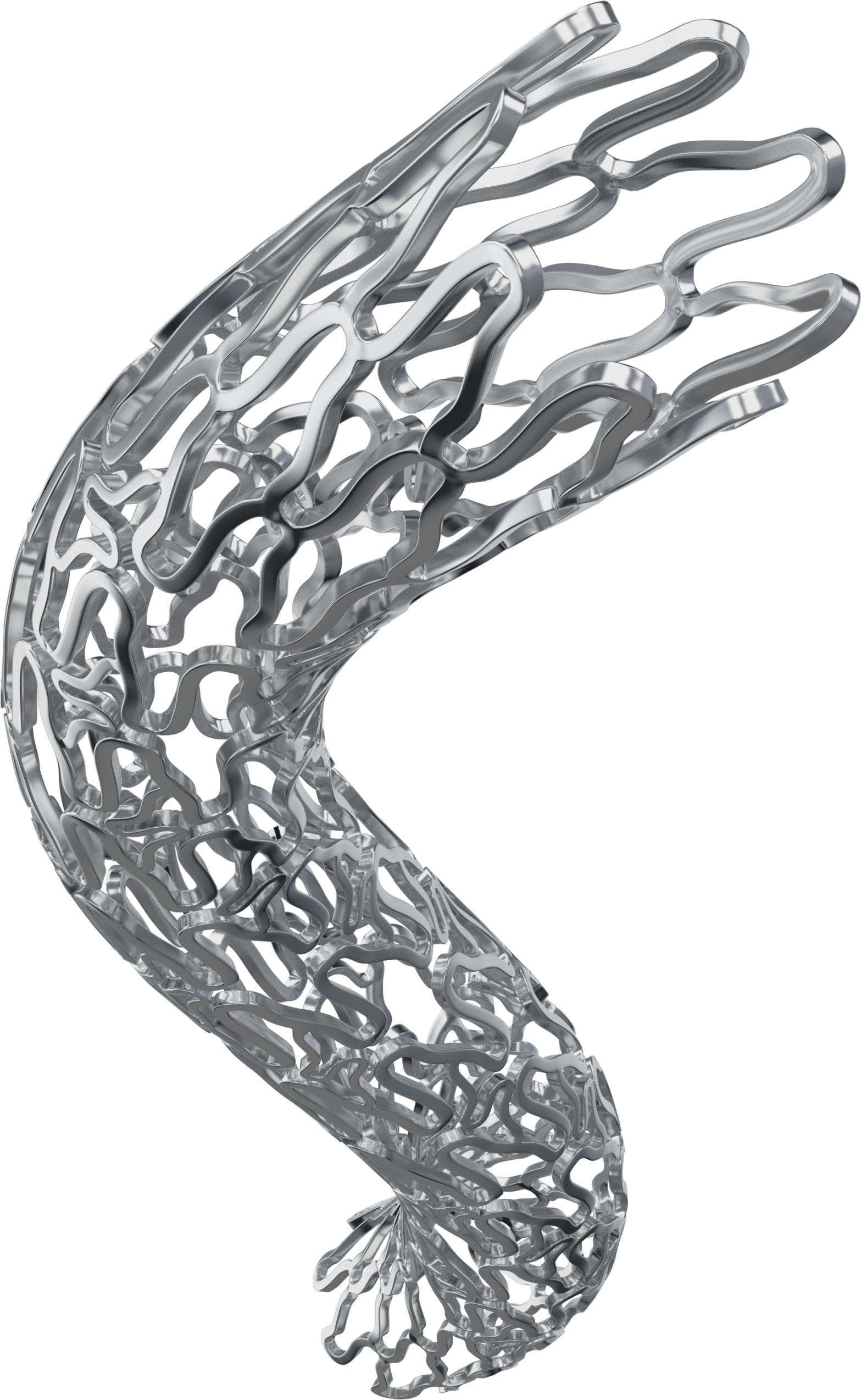
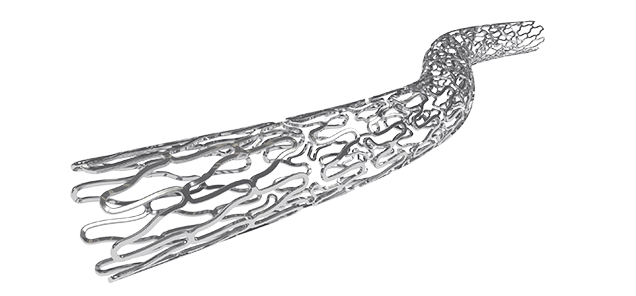
BioMimeTM is a Sirolimus Eluting Coronary Stent System with an ultra-thin (65 µm) strut thickness.
This next generation (SES) has a Novel Hybrid Design with closed cells (on both ends of the stent) & open cells (in the middle)- this allows for Morphology Mediated Expansion for adequate conformability and lesser edge dissections.
BioPoly-Biodegradable Polymer: 2µm low thickness, stable and elastic polymer.
Stent design with non-linear S links & Y connectors allowing high flexibility and adequate side branch access. Strut width variability ensures optimal radial strength.
Low balloon over hang of less than 0.5mm to minimize healthy vessel injury.
Available in more than 94 Countries worldwide with more than 500,000 stents deployed.
meriT Trials: Consistent data in more than 1,700 patients upto 5 years follow-up.
Non Inferiority to XIENCE DES proven in meriT-V: published in Eurointervention (Sep’18)
Benefits of BioMime:
- Ultra-thin 65 µm strut thickness & Biodegradable Polymer-Based DES for Faster Healing.
- Strut Width Variability for Optimal Radial Strength & Conformability.
- Morphology Mediated Expansion for Lesser Edge Dissections.
- Novel Hybrid Cell Design with Open Cells in the Middle for Adequate Side Branch Access
Visit our website to learn more about the product.